ZirChrom Newsletter vol. #6
Vol.# 6- “Rhinophase®-AB, Zirconia-based Monoclonal Antibody Purification System” (PDF)
Welcome to the sixth issue of ZirChrom's electronic newsletter. This newsletter introduces a biocompatible stationary phase useful for small and large-scale purification of biomolecules. This non-compressible media allows for high throughput and ultra-stability while maintaining unique specificity for a wide range of monoclonal antibody (Mab) subclasses.
WHAT IS RHINOPHASE®-AB?

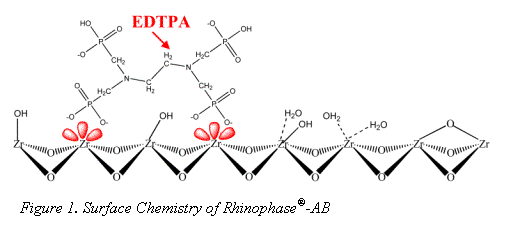
The surface chemistry of zirconia is quite different from the more traditional silica and polymer stationary phase supports. The dominant surface features of zirconia include: Brönsted acid, Brönsted base, and Lewis acid sites (see Figure 2) (1-3).
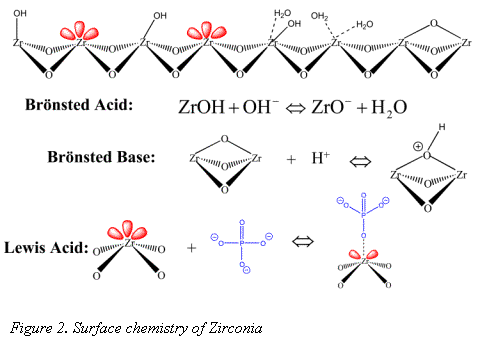
It is the Lewis acid site that sets zirconia apart from silica gel and allows for its modification with EDTPA. Without sequestering the Lewis acids sites, bare zirconia is not biocompatible (5); however modification with a hard Lewis base such as EDTPA does allow for elution of protein (4). EDTPA was chosen for its strong Lewis base and metal chelation characteristics. It has been well demonstrated that EDTPA modified zirconia may be used to selectively bind monoclonal antibody (Mab) from a cell supernatant (6). Recent work by Subramanian et. al explored the Mab/Rhinophase®-AB interaction in depth and demonstrated Rhinophase®-AB acts as a "pseudo-affinity" support for immunoglobulins (7). Pseudo-affinity supports employ certain structural binding features of proteins to establish a protein-ligand interaction. Although the exact binding mechanism of Mab on Rhinophase®-AB is still under study, Subramanian et al. have proposed that the mechanisms are mainly electrostatic and possibly hydrogen bond interactions.
WHY RHINOPHASE®-AB?
Historically the purification of Mab has been preformed using immobilized protein A or G (Staphylococcus aureus cell wall protein) on an agarose, sepharose or other resin bead. This material has many shortcomings and is far from the ideal Mab purification media. Rhinophase®-AB offers many operational advantages, especially in the following areas:
Resin Capacity
Throughput Speed
Mab Recovery
Resin Cleanability
Resin Lifetime
Mab Purity
Table 1 qualitatively compares the performance of Protein A to the revolutionary Rhinophase®-AB in terms of these six key factors. The recovery, capacity and purity of Mab purified on Rhinophase®-AB is comparable to that purified using traditional methods. However the unique chemistry and stability of the Rhinophase®-AB affords greater separation speed, cleanability, and longer media lifetime.
Table 1. Qualitative Comparison of Six Performance
Factors for Protein A or G type Gel to Rhinophase®-AB
|
|
Protein A |
Rhinophase®-AB |
|
Resin Capacity |
+ |
+ |
|
Throughput Speed |
+ |
+ + + |
|
Mab Recovery |
+ |
+ |
|
Resin Cleanability |
+ |
+ + + |
|
Resin Lifetime |
+ |
+ + |
|
Mab Purity |
+ |
+ |
+ = good, + + = very good, + + + = excellent
The following is a detailed analysis of the 6 key
operational parameters:
1. Resin Capacity:
Recent work by Subramanian et al. (7) has shown that the maximum static binding capacity for hIgG on Rhinophase®-AB to be from 55 to 65 mg IgG/ml beads. This capacity was unaffected by temperature and is comparable to that of Protein A based media. The maximum dynamic binding capacity was found to be in the range of 12 - 20 mg IgG/mL beads. Furthermore, it was shown that a buffer pH of 5.5 maximized ionic interactions and provided the maximum binding capacity for IgG.
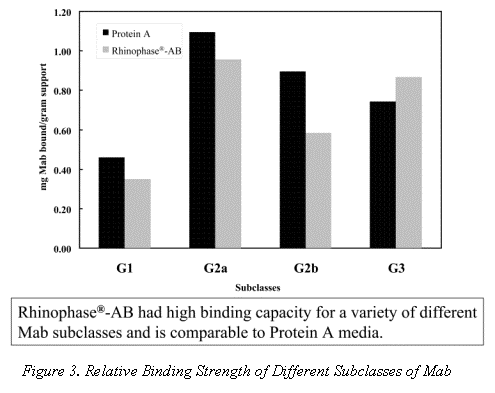
The relative dynamic binding strength of Rhinophase®-AB versus Protein A for different IgG subclasses is shown in Figure 3. In each case identical amounts of Mab were offered to the purification media in a batch mode. This shows the relative (not absolute) binding strength of each subclass for both Protein A and Rhinophase®-AB medias. In general the Rhinophase®-AB materials shows as good a binding strength for different IgG subclasses as Protein A media. All the antibodies were diluted or resuspended (as the pure Mab is in solid form) in the loading buffer (20 mM MES, 4 mM EDTPA, 50 mM NaCl, pH 5.5). For each experiment, antibodies were contacted with Rhinophase®-AB at 4 oC for 24 hours with end-to-end rotation. At the completion, the beads were settled with gentle centrifugation. The OD at 280 nm of the supernatant was measured. In most cases, the supernatant was pipetted off and the beads were washed with loading buffer. The amount of antibody bound to the support was found by difference. About 150 microliters of hydrated beads and 400 microliters of Mab solution were used to do this work. Due to the expensive nature of the samples, concentrations of Mab were kept between 1 to 2 mg/ml in the experiments using pure IgG2a, IgG2b and IgG3. Protein A was purchased from Pierce Chemical Company Rockford, Illinois.
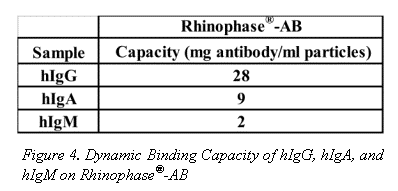
Dynamic binding capacities of other immunoproteins, of differing molecular weights, on Rhinophase®-AB are shown in Figure 4. Values in Figure 4 are reported as an average of 3-independent replicate experiments. The standard deviation is less than 5%. All capacities are reported as mg Ig bound per ml of beads.
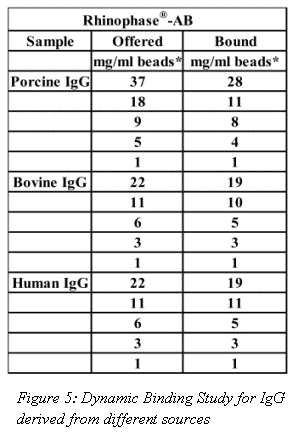
Dynamic binding studies for IgG derived from different animal sources are shown in Figure 5. In general, Rhinophase®-AB showed a very high affinity for all IgG, but was more retentive of Bovine and Human IgG.
2. Throughput Speed:
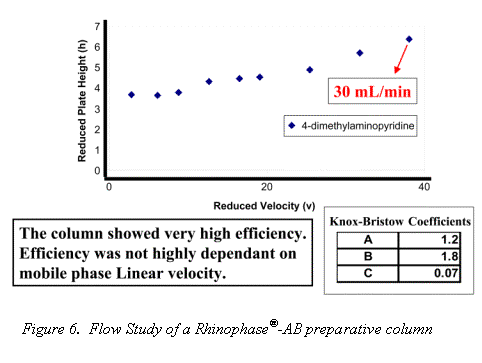
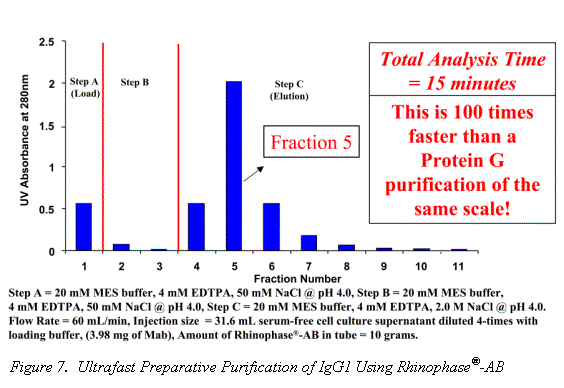
Traditional Mab purification methods have been based on polymer and agarose resins. The inherent compressibility of these gel type phases prohibits their use at higher flow rates. Alternatively, the excellent mechanical strength and mass transfer characteristics of the Rhinophase®-AB zirconia stationary phase allows for Mab purifications to be performed at high mobile phase linear velocities without large losses in column efficiency (See Figures 6 & 7).
3. Mab Recovery:
Clausen et al (6) measured yields by ELISA to fall within 92-98% with little or no detectable Mab in column fall-through and wash fractions. Typical yields of Mab purifications on Rhinophase®-AB are greater than 95% with purity levels equal to or greater than affinity gel-type media. Typically a higher pH (4.0 - 5.5) than used with Protein A is used to elute antibody from Rhinophase®-AB. This reduces the likelihood of denaturing and loss of biological activity often associated with the acidic elution regimes.
4. Resin Cleanability:
Zirconia is extremely
resilient to chemical degradation.
Subramanian et al. (7) demonstrated that the EDTPA-modification is
stable from pH 1 to 10 and the zirconia particle itself is stable over the
entire pH range. This stability
prevents degradation from microbial attack and allows for aggressive
regeneration after column “fouling”. On
the other hand, fragile Protein A can degrade when exposed to harsh cleaning
conditions causing decreases in capacity and leaching of biological ligand into
the final Mab product.
5. Resin Lifetime:
The stability of the
Rhinophase®-AB zirconia media and EDTPA modification allow for
extremely long operation lifetime using recommended operating conditions. In addition, Rhinophase®-AB will
not leach antigen into purified antibody, an often reported issue with aging
affinity or Protein A resins.
Rhinophase®-AB is not an antigen-based affinity resin nor is
its ligand from a biological source.
6. Mab Purity:
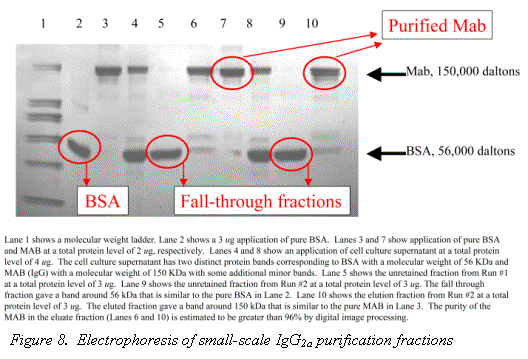
The purity of Rhinophase®-AB purified Mab from a cell culture supernatant has been estimated using digital image processing of an ELISA to be greater than 98% (6). Figure 8, shows the an SDS PAGE gel electropherogram of a IgG2a purification using Rhinophase®-AB; the purity was estimated by digital image processing to be greater than 96%.
SUMMARY:
The superior stability of Rhinophase®-AB results in superior speed, cleanability, and lifetime; while maintaining the capacity, recovery and purity of traditional affinity purification technologies.
Rhinophase®-AB is available in bulk, in
packed column, or in research and production kits complete with the items necessary to perform laboratory
scale purifications (just add water!) (Figure 9 & 10). Please contact a ZirChrom Technical
Specialist at (1-866-STABLE-1 or support@zirchrom.com) today to obtain more
information or to discuss your specific purification needs.

REFERENCES:
(1) Blackwell, J. A.; Carr, P. W. Journal of Liquid Chromatography 1991, 14, 2875-2889.
(2) Blackwell, J. A.; Carr, P. W. Analytical Chemistry 1992, 64, 853-862.
(3) Rigney, M.P.; Funkenbusch, E.F.; Carr, P.W. Journal of Chromatography 1990, 499, 291-304
(4) Clausen, A. M.; Carr, P. W. Analytical Chemistry 1998, 70, 378-385.
(5) Carr, P. W.; Blackwell, J. A.; Weber, T. P.; Schafer, W. A.; Rigney, M. P. ACS Symposium Series 1993, 529, 146-164.
(6) Clausen, A. M.; Subramanian, A.; Carr, P. W. Journal of Chromatography, A 1999, 831, 63-72.
(7) Sarkar, S.; Carr, P. W.; McNeff, C. V.; Subramanian, A. Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences 2003, 790, 143-152.