ZirChrom Newsletter Vol. #14
Vol.# 14- “Multi-modal Separations Case Study: Separation of Positional Aromatic Amine Isomers using Sub-2micron ZirChrom®-PBD” (PDF)
WHY CHOOSE ZIRCONIA FOR THE ANALYSIS OF POSITIONAL
ISOMERS?
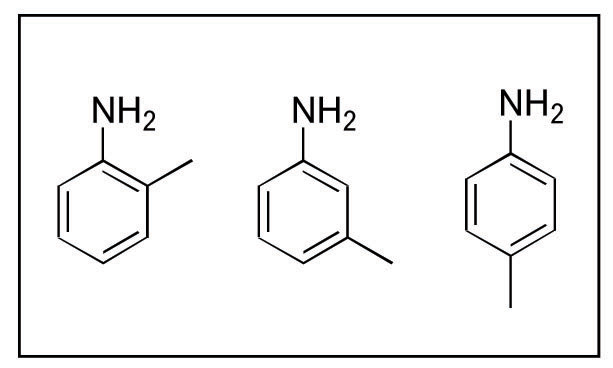
Under normal operation conditions for typical silica columns the separation of amines can be complex. Add the additional complication of the positional isomers, such as in the analysis of o-, m-, p- toluidine (see Figure 1), and the problem becomes infinitely more complex. In this newsletter the method development for this set of compounds will be outlined in detail with special attention paid to the use of the unique properties of zirconia to “tune” in an optimal separation for these three positively charged, positional isomers.

Figure 1. o-, m-, p- Toluidine
Zirconia’s basic chemical structure offers unparalleled chemical and thermal stability. The crystalline structure of zirconia has seven bond valency when compared to silica’s four. The additional bonds in the crystalline structure of zirconia protect the structure from the hydrolysis that causes silica to degrade at low and high pH. The result of this unique configuration is a particle with superior pH (1-14) and temperature (up to 200ºC) stability. This structure also enables a unique surface chemistry. The surface of zirconia has highly reproducible Lewis acid, Bronsted base, and Bronsted acid sites (see Figure 2).
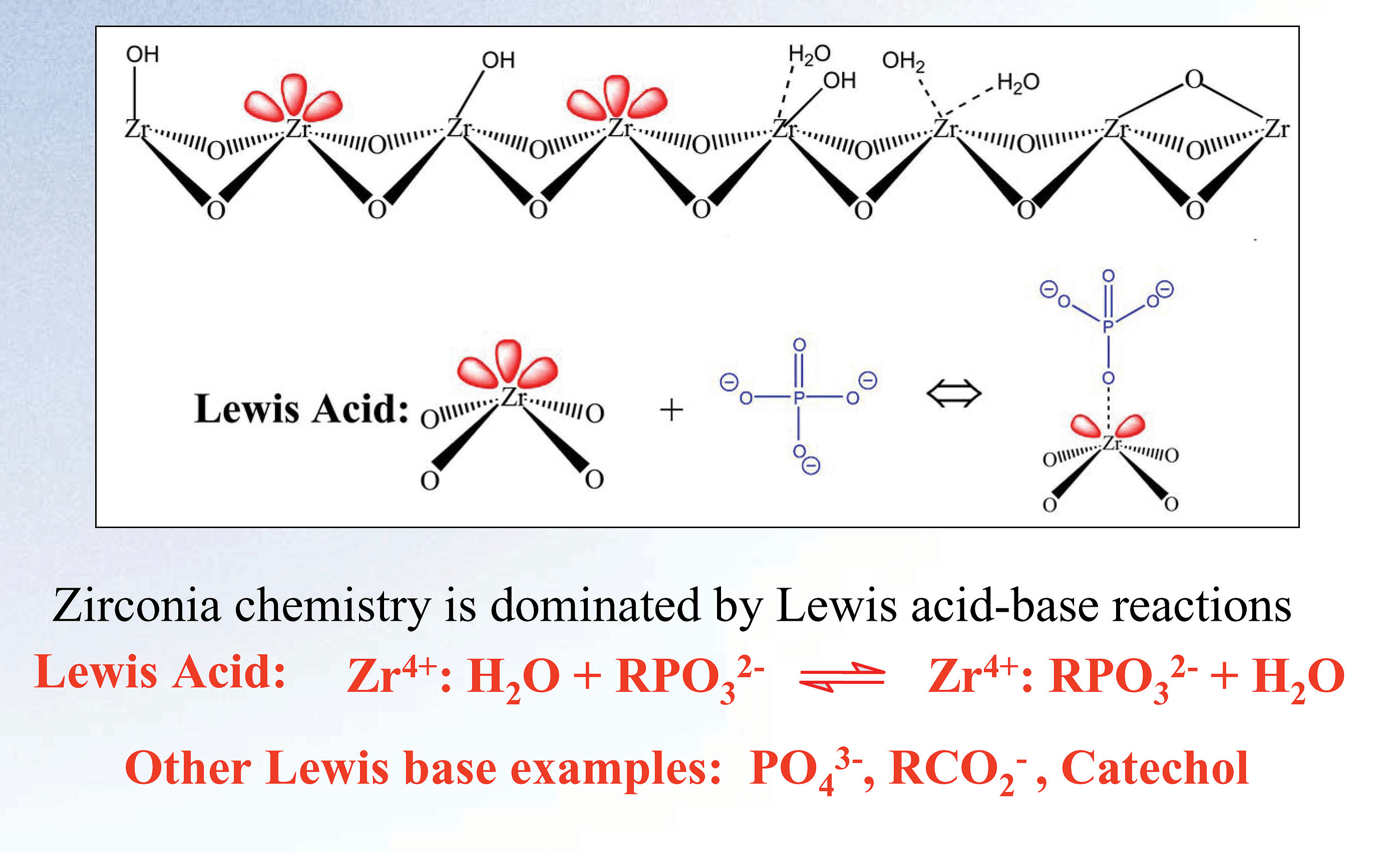
Figure 2. Surface Chemistry of Zirconia
Although the benefits of zirconia’s unparalleled stability are clearly apparent, the more subtle value of the multi-modal nature of zirconia may in fact be the most important factor when considering a zirconia column for your application.
SURFACE CHEMISTRY OF ZIRCHROM®-PBD
FOR MULTIMODE SEPARATION OF CATIONS
The ZirChrom®-PBD phase is coated with cross-linked polybutadiene (see Figure 3).
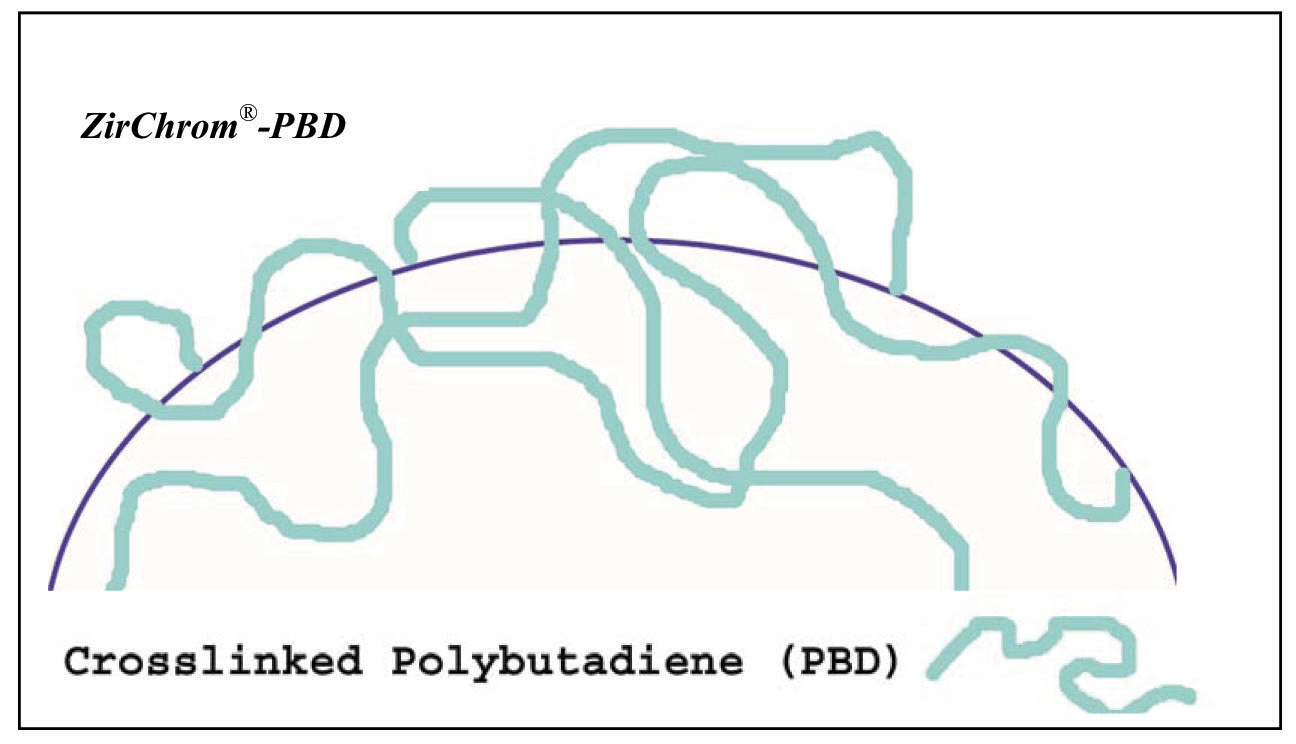
Figure 3. Surface Chemistry of ZirChrom®-PBD
The Lewis acid sites on the surface of ZirChrom®-PBD allow for a tunable, multi-modal, selectivity. Without the addition of any Lewis base modifiers in the mobile phase the surface of zirconia is positive at low pH, neutral at neutral pH and negative at high pH (See Figure 4).
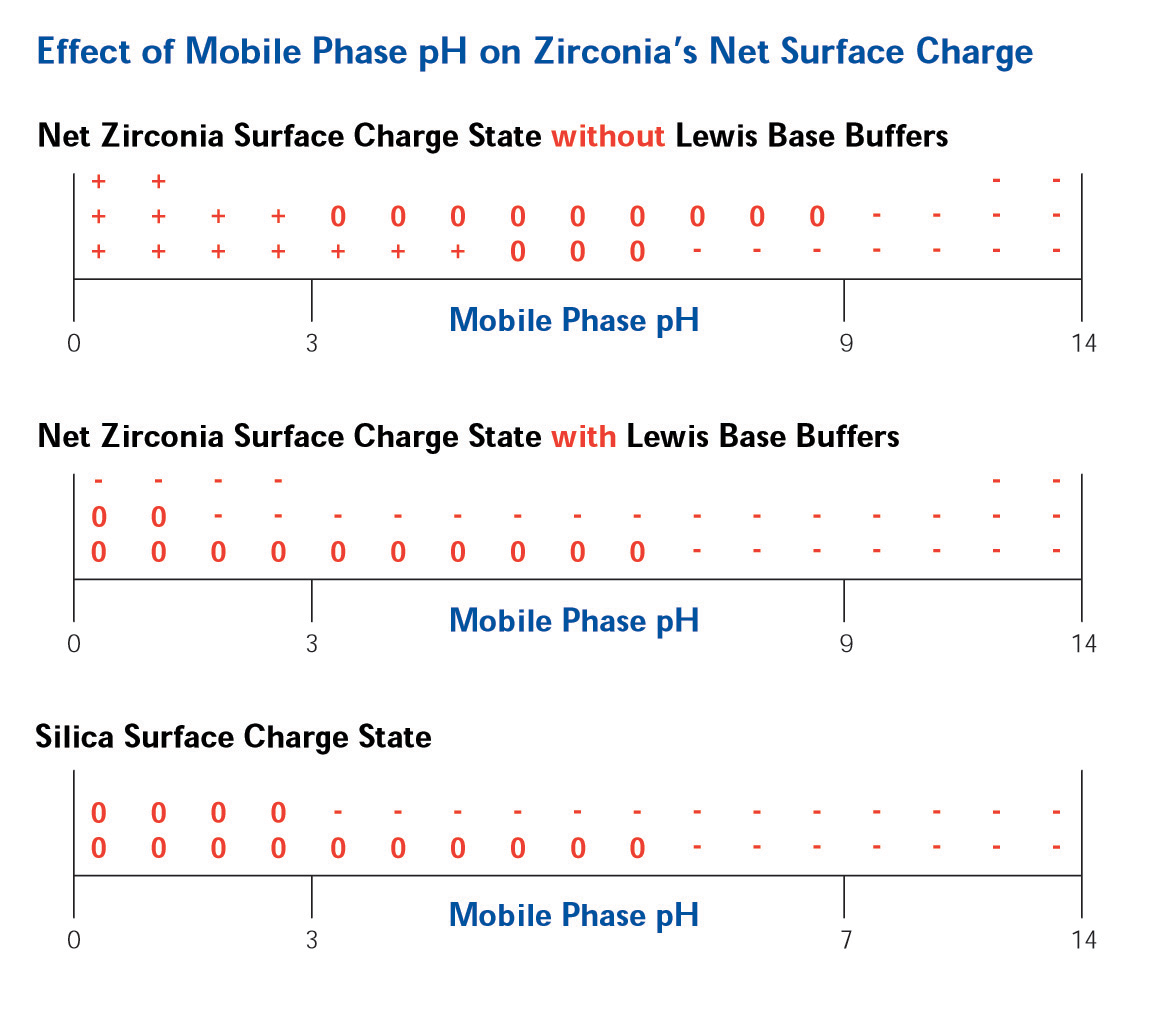
Figure 4. Effect of Mobile Phase pH on Surface Charge
The addition of a Lewis base modifier (such as carboxylate or phosphate) to the mobile phase will cause the modifier to adsorb to the zirconia surface in a ligand exchange interaction with the Lewis acid sites on the surface. The magnitude of the net negative charge imparted on the surface is directly correlated with the strength of the Lewis base modifier on the eluotropic scale (See Figure 5).
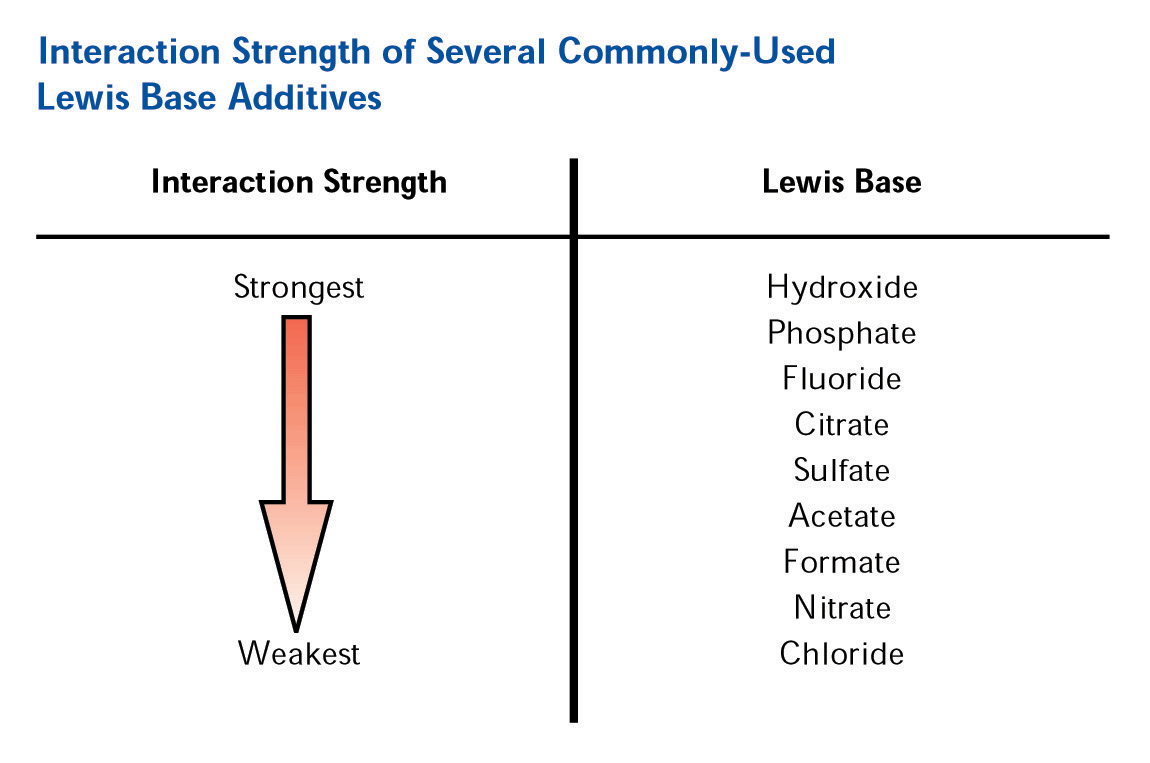
Figure
5. Eluotropic Scale for Lewis Base Modifiers
The negative charge imparted on the surface (with phosphate or EDTPA a net negative charge is possible even at very low pH) coupled with the reversed phase character of the polymer coating and unparalleled thermal and chemical stability allows for a very unique multi-mode separation by cation exchange and reversed phase. Furthermore by judiciously selecting the mobile phase modifier and separation pH, the extent each mode (SCX or RP) plays in the separations can be controlled. This enables users to “tune” the separation, allowing for effortless optimization of the analysis. It is exactly this feature of the ZirChrom-PBD phase that we put to full advantage in the method development for these aromatic amine isomers.
EFFECT OF PH ON MULTIMODE
SELECTIVITY ON ZIRCHROM®-PBD
In Figure 6 the effect of pH changes on the separation of o-, m-, p- toluidine is examined.
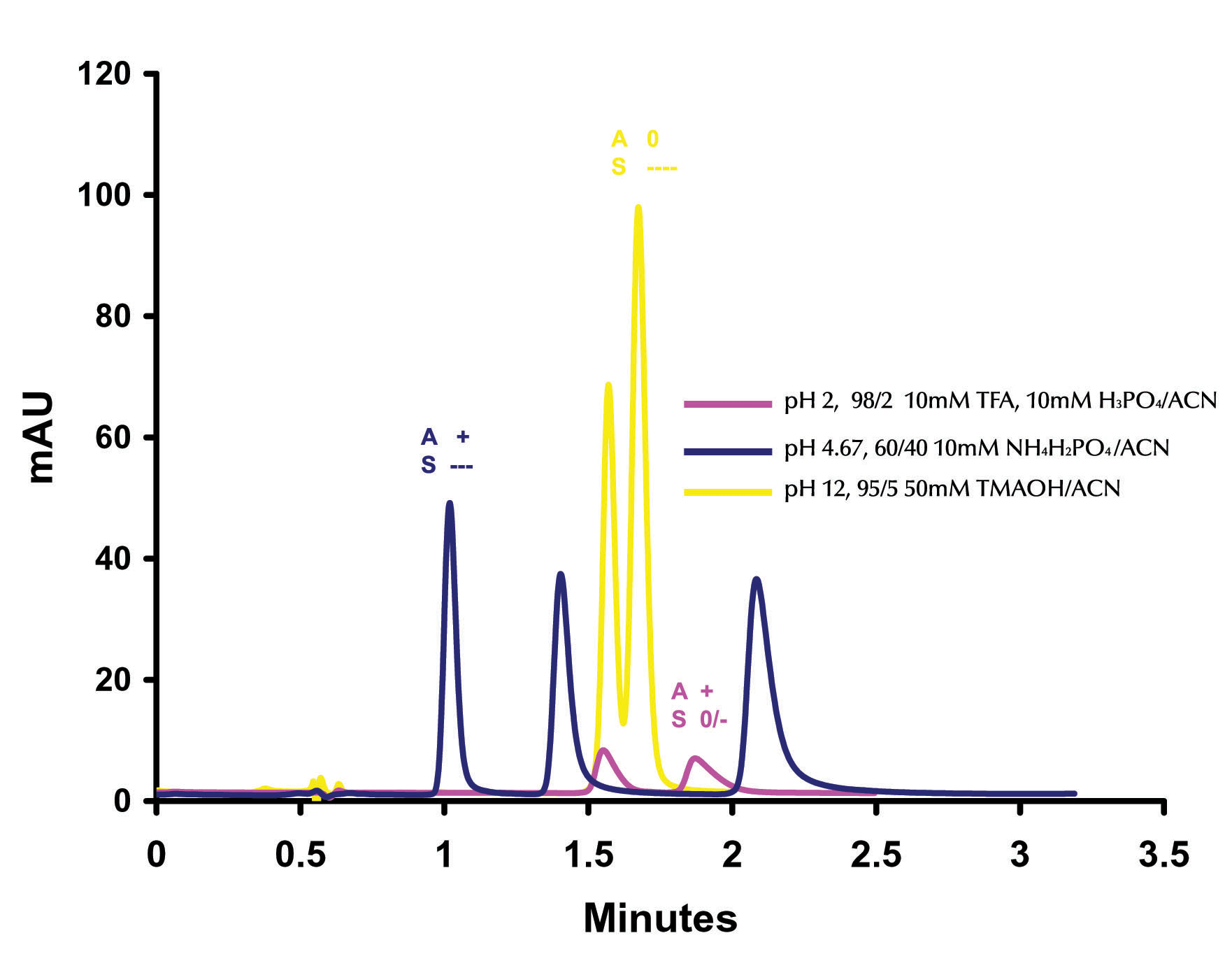
Figure 6. Effect of pH on the Separation of o-, m-, p- Toluidine ZirChrom®-PBD. 50 mm x 4.6 mm i.d (P/N ZR03-0546); Mobile Phase: As Indicated; Particle Size: 3micron; Flow Rate: 1 ml/min; Temperature: 25ºC; Detection by UV @ 254 nm; Instrument: Agilent 1100.
For each pH condition both the hypothetical analyte net
charge (A) and the hypothetical surface net charge (S) are displayed.
At very high pH (yellow trace) we expect to see a primarily reversed
phase separation as the net charge on the surface of the zirconia is very
negative and the analytes should be neutral (i.e. no ion exchange contribution).
At very low pH (mauve trace) we would again expect to see a primarily reversed
phase interaction as the surface carries a net neutral to weakly negative charge
and the analytes would be positively charged (i.e. minimal ion exchange
contribution). In Figure 6 we can
see that this is indeed the case with the yellow and mauve chromatographs having
similar retention times and selectivity (only two peaks).
Slightly more retention and significantly more tailing are apparent on
the low pH chromatogram, this can be attributed to the small amount of negative
charge on the surface of the particle causing a minimal and uncontrolled ion
exchange contribution. To achieve an effective multi-mode separation,
conditions must be isolated at which both the surface and the analyte carry
opposing charges which can significantly contribute to the retention and
separation of the analytes by ion exchange.
At pH 4.67 (blue trace) with phosphate the net charge on the surface of
the zirconia particle is negative (due to the adsorption of the charged
phosphate on surface Lewis acid sites) and the analytes are positively charged.
Under this condition all three of the compounds (o-, m-, p-
toluidine) are baseline resolved within 3 minutes.
EFFECT OF IONIC STRENGTH ON TAILING OF AROMATIC AMINES
The initial pH scouting work was done on a 3micron particle column. Once the optimum buffer (phosphate) and pH (4.6) were determined these conditions were then transferred onto a ZirChrom®-PBD sub-2micron phase column, (Figure 7).
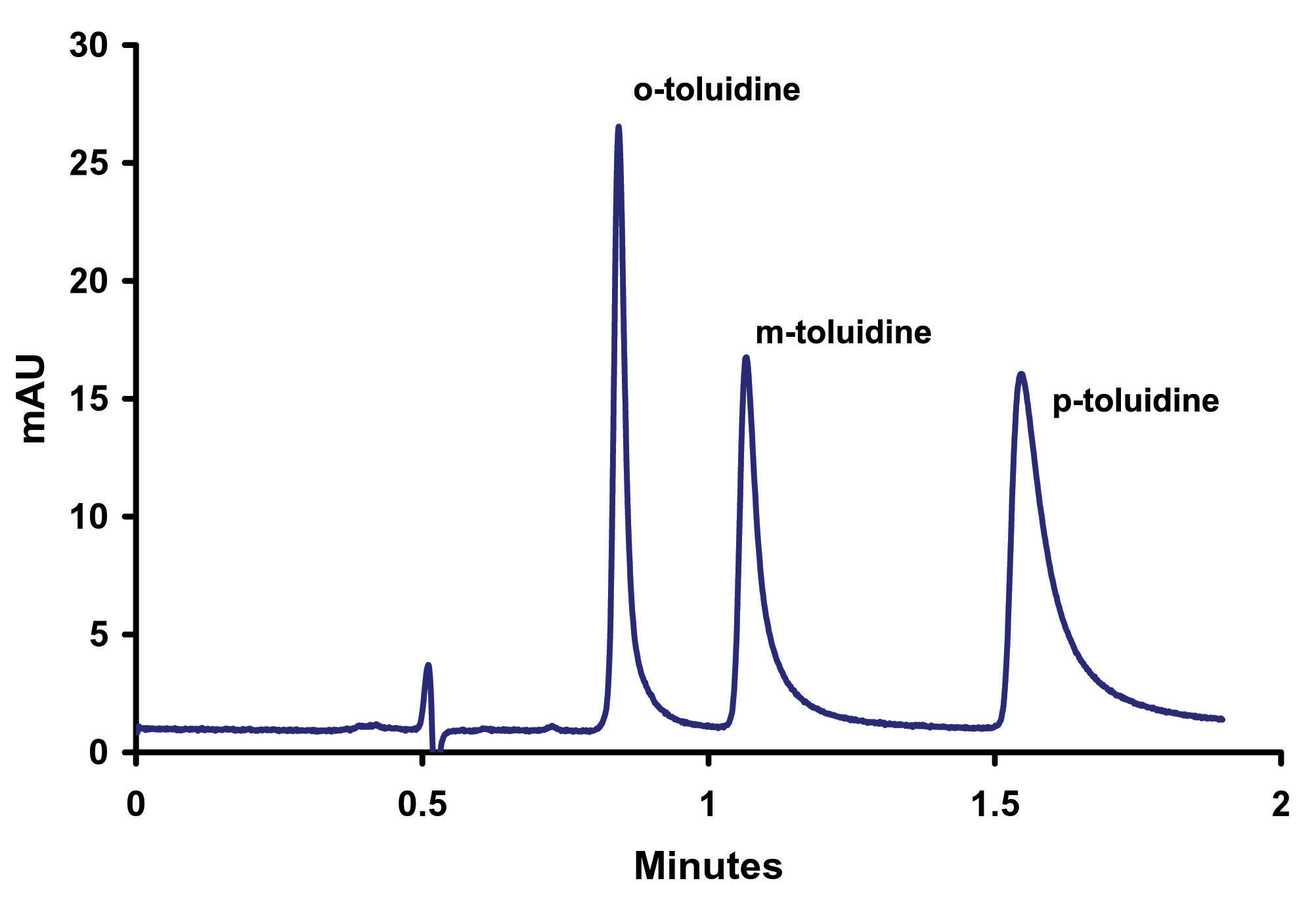
Figure 7. Separation of o-, m-, p- Toluidine on Sub-2micron ZirChrom®-PBD, 50 mm x 4.6mm i.d, (P/N ZR03-0546-1.9); Mobile Phase: 35/65 ACN/10mM NH4H2PO4, pH=4.6; Flow Rate: 1.0 ml/min; Temperature: 25ºC; Back Pressure: 214 bar; Detection by UV @ 254 nm; Instrument: Agilent 1100.
This resulted in a much faster separation; achieving baseline resolution in less than 2 minutes. However, the peaks, especially p-toluidine, appear to exhibit increased tailing. We hypothesize that this increased tailing is due to an uncontrolled ionic interaction. To test this hypothesis the ionic strength of the mobile phase was increased while keeping all other variables constant (Figure 8).
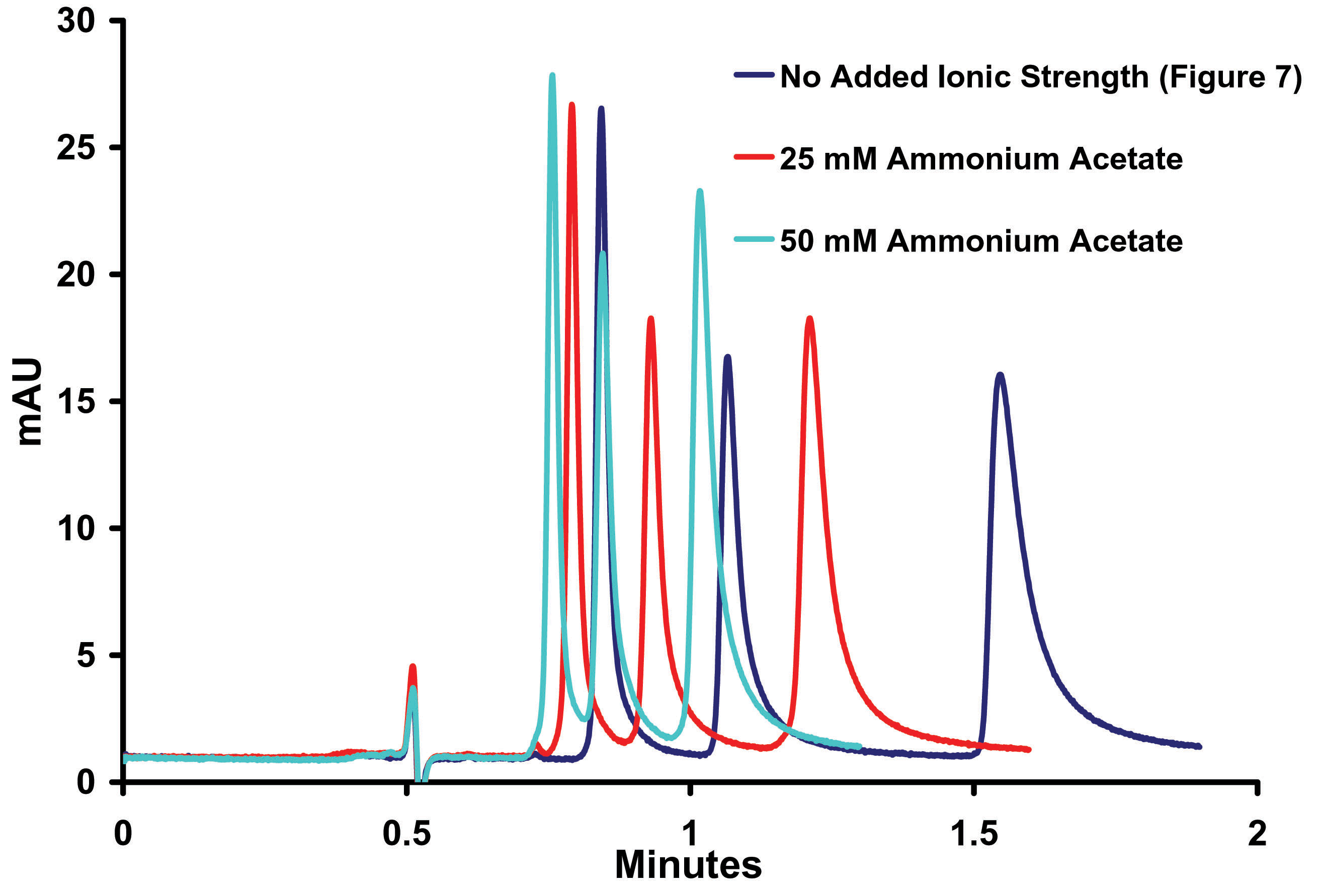
Figure
8. Effect
of Ionic Strength on the Separation o-, m-, p- Toluidine on Sub-2micron
ZirChrom®-PBD, 50mm x 4.6mm i.d, (P/N ZR03-0546-1.9); Mobile Phase:
35/65 ACN/10mM NH4H2PO4, pH=4.6: Flow Rate: 1.0
ml/min; Temperature: 25º;
Back Pressure 216 bar;
Detection by UV @ 254 nm; Instrument: Agilent 1100
The data presented in Figure 8 confirms that ion exchange is playing a large role in this separation and, as expected, as the ionic strength increases both tailing and retention decrease. The addition of 25mM ammonium acetate appears to provide the most benefit to peak shape without loss of resolution and was chosen for further research.
TEMPERATURE AND ORGANIC ADJUSTMENT
The chromatographer’s ability to make practical use of the promised efficiency and speed benefits of sub-2micron particles has long been hindered by the need for specialized high pressure instrumentation. ZirChrom®-PBD is stable up to temperatures up to 150 °C. The decrease in mobile phase viscosity provided by high temperature is especially important to overcome the higher back pressures inherent with sub-2micron particle columns. This decrease, coupled with the unique multi-mode selectivity of ZirChrom®-PBD, enables the user to take full advantage of the increased efficiency provided by the smaller particles without the use of specialized UHPLC instrumentation (Figure 9).
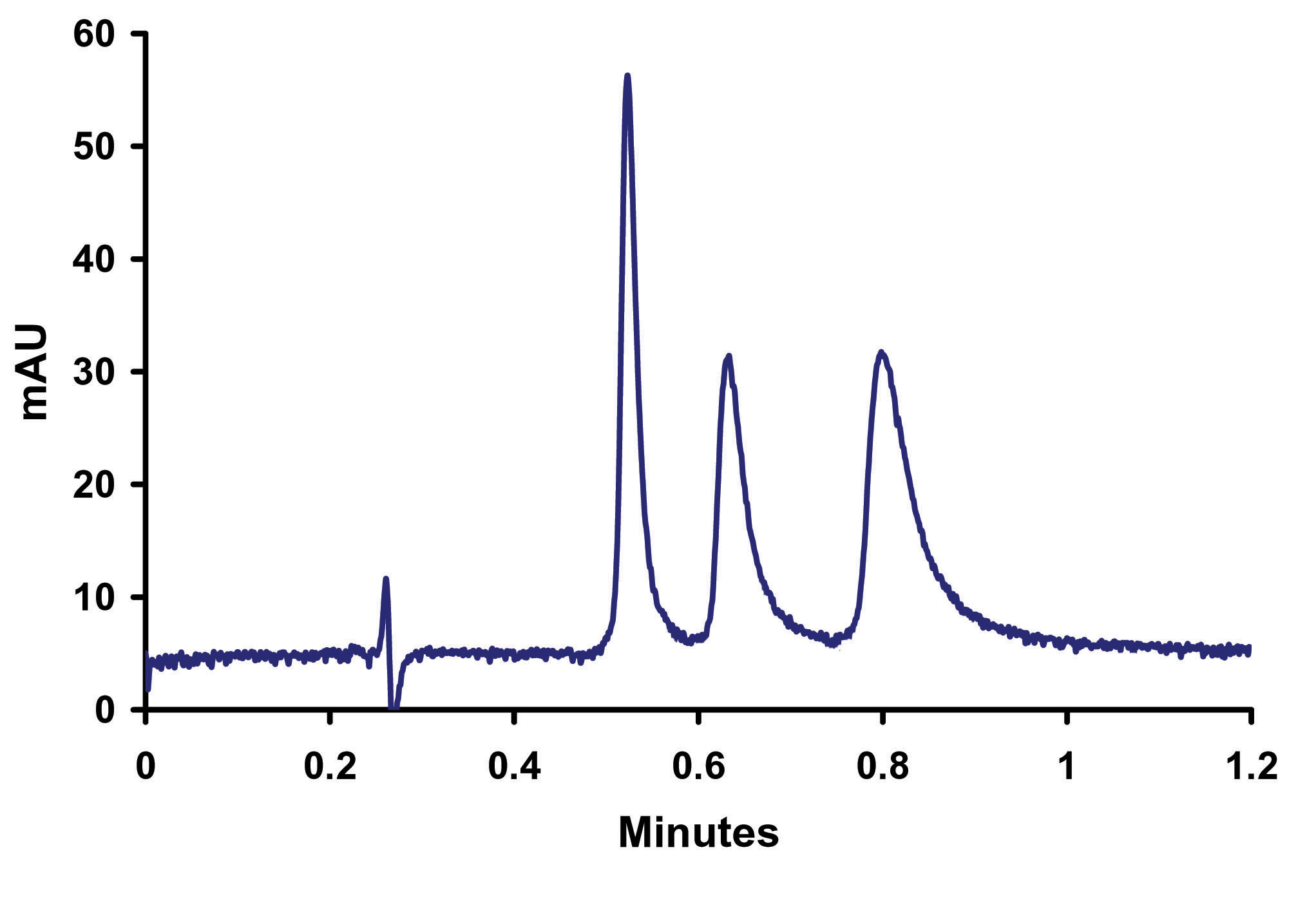
Figure 9. Elevated Separation o-, m-, p- Toluidine on Sub-2micron ZirChrom®-PBD, 50mm x 4.6mm i.d (P/N ZR03-0546-1.9); Mobile Phase: 10/90 ACN/10mM NH4H2PO4, 25mM NH4OAc pH=4.6; Flow Rate: 2 ml/min; Temperature: 80ºC; Back Pressure 208 bar; Detection by UV @ 254 nm; Instrument: Agilent 1100.
In Figure 9 the increase in temperature to 80ºC enables a two-fold increase in flow rate without an increase in back pressure thus allowing for the baseline resolution of the three toludine positional isomers in less than a minute. In fine-tuning the separation decreases in organic content, to compensate for the increased elution strength of water at the higher temperature, were required to maintain the resolution of the compounds. The organic requirement for the separation is further reduced by the shorter run time, allowing for twice the savings on solvent purchase and disposal.
In this note we have demonstrated that the ZirChrom®-PBD sub-2micron particle columns are versatile, stable and have a unique selectivity. A ZirChrom column facilitates the use of all the method development tools (temperature, ionic Strength, pH, organic) available on most standard HPLC instrumentation to achieve optimal separation without fear of column degradation. This method, and other applications on our website, can be tailored to your specific application needs. ZirChrom technical support can help to optimize and transfer this method to your site. Please contact ZirChrom technical support at 1-866-STABLE-1 or support@zirchrom.com for details.
Additional examples of multi-mode separations are available on our website at: http://www.zirchrom.com/multimode.asp. Additional information regarding the new sub-2micron particles is available on our website at: http://www.zirchrom.com/UHPLC.asp. Our next installment of Z-APPS will feature recent multi-modal sub-2micron research.